Search
- Page Path
- HOME > Search
- Diabetes, Obesity and Metabolism
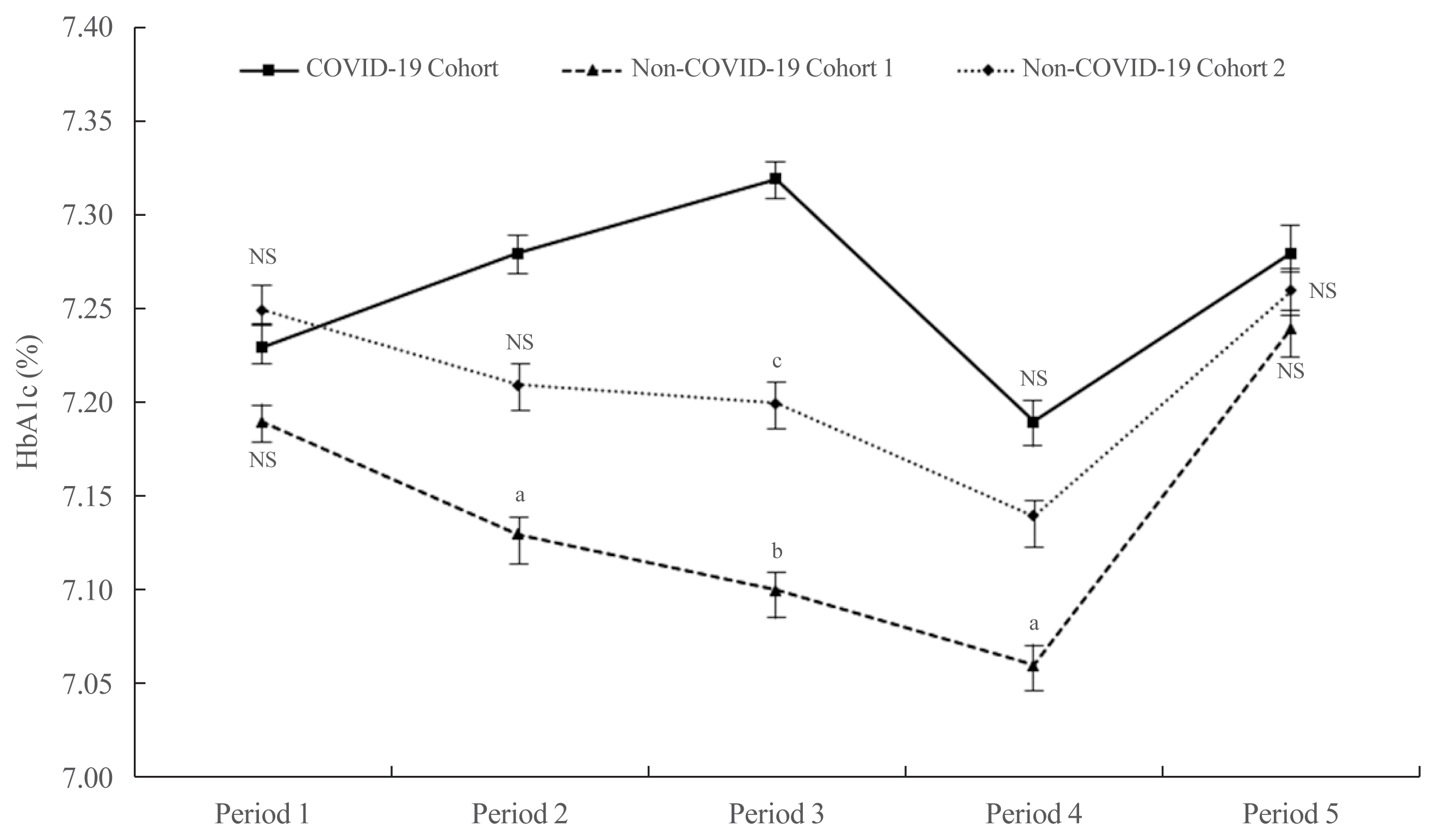
- Year-Long Trend in Glycated Hemoglobin Levels in Patients with Type 2 Diabetes during the COVID-19 Pandemic
- Jonghwa Jin, Seong Wook Lee, Won-Ki Lee, Jae-Han Jeon, Jung-Guk Kim, In-Kyu Lee, Yeon-Kyung Choi, Keun-Gyu Park
- Endocrinol Metab. 2021;36(5):1142-1146. Published online October 21, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1154
- 3,884 View
- 148 Download
- 4 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - It has been suggested that the coronavirus disease 2019 (COVID-19) pandemic has had a negative impact on glycemic control in patients with type 2 diabetes mellitus (T2DM). However, no study has examined yearly trends in glycated hemoglobin (HbA1c) levels after the start of the COVID-19 outbreak. Here, we performed a retrospective analysis of HbA1c concentrations during the early period of the COVID-19 outbreak (COVID-19 cohort) and then compared the yearly trend in the mean HbA1c level, along with fluctuations in HbA1c levels, with those during previous years (non-COVID-19 cohorts). We observed that the mean HbA1c level in patients with T2DM increased during the first 6 months of the COVID-19 outbreak. After 6 months, HbA1c levels in the COVID-19 cohort returned to levels seen in the non-COVID-19 cohorts. The data suggest that vulnerable patients with T2DM should be monitored closely during the early period of a pandemic to ensure they receive appropriate care.
-
Citations
Citations to this article as recorded by- Physical and Mental Health Characteristics of Hospitalized COVID-19 Patients with and without Type 2 Diabetes Mellitus in Turkey
Abdulbari Bener, Murat Atmaca, Abdulla O. A. A. Al-Hamaq, Antonio Ventriglio
Brain Sciences.2024; 14(4): 377. CrossRef - A Hybrid Model of In-Person and Telemedicine Diabetes Education and Care for Management of Patients with Uncontrolled Type 2 Diabetes Mellitus: Findings and Implications from a Multicenter Prospective Study
Ayla M. Tourkmani, Turki J. Alharbi, Abdulaziz M. Bin Rsheed, Azzam F. Alotaibi, Mohammed S. Aleissa, Sultan Alotaibi, Amal S. Almutairi, Jancy Thomson, Ahlam S. Alshahrani, Hadil S. Alroyli, Hend M. Almutairi, Mashael A. Aladwani, Eman R. Alsheheri, Hyfa
Telemedicine Reports.2024; 5(1): 46. CrossRef - The indirect impact of the COVID-19 pandemic on people with type 2 diabetes mellitus and without COVID-19 infection: Systematic review and meta-analysis
Zhuoran Hu, Hin Moi Youn, Jianchao Quan, Lily Luk Siu Lee, Ivy Lynn Mak, Esther Yee Tak Yu, David Vai-Kiong Chao, Welchie Wai Kit Ko, Ian Chi Kei Wong, Gary Kui Kai Lau, Chak Sing Lau, Cindy Lo Kuen Lam, Eric Yuk Fai Wan
Primary Care Diabetes.2023; 17(3): 229. CrossRef - Evaluating Effects of Virtual Diabetes Group Visits in Community Health Centers During the COVID-19 Pandemic
Tracy Dinh, Erin M Staab, Daisy Nuñez, Mengqi Zhu, Wen Wan, Cynthia T Schaefer, Amanda Campbell, Michael Quinn, Arshiya A Baig
Journal of Patient Experience.2023;[Epub] CrossRef - Cardiovascular-related health behavior changes: lessons from the COVID-19 pandemic and post-pandemic challenges
Inha Jung, Won-Young Lee
Cardiovascular Prevention and Pharmacotherapy.2023; 5(4): 99. CrossRef
- Physical and Mental Health Characteristics of Hospitalized COVID-19 Patients with and without Type 2 Diabetes Mellitus in Turkey

- Lobeglitazone, a Novel Peroxisome Proliferator-Activated Receptor γ Agonist, Attenuates Renal Fibrosis Caused by Unilateral Ureteral Obstruction in Mice
- Kwi-Hyun Bae, Jung Beom Seo, Yun-A Jung, Hye-Young Seo, Sun Hee Kang, Hui-Jeon Jeon, Jae Man Lee, Sungwoo Lee, Jung-Guk Kim, In-Kyu Lee, Gwon-Soo Jung, Keun-Gyu Park
- Endocrinol Metab. 2017;32(1):115-123. Published online February 28, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.1.115
- 4,830 View
- 77 Download
- 13 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Renal tubulointerstitial fibrosis is a common feature of the final stage of nearly all cause types of chronic kidney disease. Although classic peroxisome proliferator-activated receptor γ (PPARγ) agonists have a protective effect on diabetic nephropathy, much less is known about their direct effects in renal fibrosis. This study aimed to investigate possible beneficial effects of lobeglitazone, a novel PPARγ agonist, on renal fibrosis in mice.
Methods We examined the effects of lobeglitazone on renal tubulointerstitial fibrosis in unilateral ureteral obstruction (UUO) induced renal fibrosis mice. We further defined the role of lobeglitazone on transforming growth factor (TGF)-signaling pathways in renal tubulointerstitial fibrosis through
in vivo andin vitro study.Results Through hematoxylin/eosin and sirius red staining, we observed that lobeglitazone effectively attenuates UUO-induced renal atrophy and fibrosis. Immunohistochemical analysis in conjunction with quantitative reverse transcription polymerase chain reaction and Western blot analysis revealed that lobeglitazone treatment inhibited UUO-induced upregulation of renal Smad-3 phosphorylation, α-smooth muscle actin, plasminogen activator inhibitor 1, and type 1 collagen.
In vitro experiments with rat mesangial cells and NRK-49F renal fibroblast cells suggested that the effects of lobeglitazone on UUO-induced renal fibrosis are mediated by inhibition of the TGF-β/Smad signaling pathway.Conclusion The present study demonstrates that lobeglitazone has a protective effect on UUO-induced renal fibrosis, suggesting that its clinical applications could extend to the treatment of non-diabetic origin renal disease.
-
Citations
Citations to this article as recorded by- The modulation effects of plant‐derived bioactive ingredients on chronic kidney disease: Focus on the gut–kidney axis
Shiyan Jian, Kang Yang, Lingna Zhang, Limeng Zhang, Zhongquan Xin, Chaoyu Wen, Shansong He, Jinping Deng, Baichuan Deng
Food Frontiers.2023; 4(1): 262. CrossRef - Druggability of lipid metabolism modulation against renal fibrosis
Yuan-yuan Chen, Xiao-guang Chen, Sen Zhang
Acta Pharmacologica Sinica.2022; 43(3): 505. CrossRef - Lobeglitazone attenuates fibrosis in corneal fibroblasts by interrupting TGF-beta-mediated Smad signaling
Selikem Nuwormegbe, Na-Young Park, Sun Woong Kim
Graefe's Archive for Clinical and Experimental Ophthalmology.2022; 260(1): 149. CrossRef - Comparative Efficacy of Lobeglitazone Versus Pioglitazone on Albuminuria in Patients with Type 2 Diabetes Mellitus
Kyung-Soo Kim, Sangmo Hong, Hong-Yup Ahn, Cheol-Young Park
Diabetes Therapy.2021; 12(1): 171. CrossRef - Lobeglitazone: A Novel Thiazolidinedione for the Management of Type 2 Diabetes Mellitus
Jaehyun Bae, Taegyun Park, Hyeyoung Kim, Minyoung Lee, Bong-Soo Cha
Diabetes & Metabolism Journal.2021; 45(3): 326. CrossRef - Lobeglitazone, A Peroxisome Proliferator-Activated Receptor-Gamma Agonist, Inhibits Papillary Thyroid Cancer Cell Migration and Invasion by Suppressing p38 MAPK Signaling Pathway
Jun-Qing Jin, Jeong-Sun Han, Jeonghoon Ha, Han-Sang Baek, Dong-Jun Lim
Endocrinology and Metabolism.2021; 36(5): 1095. CrossRef - Protocol for a preclinical systematic review and meta-analysis of pharmacological targeting of peroxisome proliferator-activated receptors in experimental renal injury
William P Martin, Yeong H D Chuah, Emer Conroy, Alison L Reynolds, Conor Judge, Francisco J López-Hernández, Carel W le Roux, Neil G Docherty
BMJ Open Science.2021;[Epub] CrossRef - Stevioside inhibits unilateral ureteral obstruction‐induced kidney fibrosis and upregulates renal PPARγ expression in mice
Wei Shen, Ke Fan, Ying Zhao, Junyan Zhang, Meilin Xie
Journal of Food Biochemistry.2020;[Epub] CrossRef - FBW7 Regulates the Autophagy Signal in Mesangial Cells Induced by High Glucose
Chenlin Gao, Fang Fan, Jiao Chen, Yang Long, Shi Tang, Chunxia Jiang, Yong Xu
BioMed Research International.2019; 2019: 1. CrossRef - Treatment with Lobeglitazone Attenuates Hepatic Steatosis in Diet-Induced Obese Mice
Sorim Choung, Kyong Hye Joung, Bo Ram You, Sang Ki Park, Hyun Jin Kim, Bon Jeong Ku
PPAR Research.2018; 2018: 1. CrossRef - VCE‐004.3, a cannabidiol aminoquinone derivative, prevents bleomycin‐induced skin fibrosis and inflammation through PPARγ‐ and CB2 receptor‐dependent pathways
Carmen del Rio, Irene Cantarero, Belén Palomares, María Gómez‐Cañas, Javier Fernández‐Ruiz, Carolina Pavicic, Adela García‐Martín, Maria Luz Bellido, Rafaela Ortega‐Castro, Carlos Pérez‐Sánchez, Chary López‐Pedrera, Giovanni Appendino, Marco A Calzado, Ed
British Journal of Pharmacology.2018; 175(19): 3813. CrossRef - EHP-101, an oral formulation of the cannabidiol aminoquinone VCE-004.8, alleviates bleomycin-induced skin and lung fibrosis
Adela García-Martín, Martín Garrido-Rodríguez, Carmen Navarrete, Carmen del Río, María L. Bellido, Giovanni Appendino, Marco A. Calzado, Eduardo Muñoz
Biochemical Pharmacology.2018; 157: 304. CrossRef - Effects of Lobeglitazone, a New Thiazolidinedione, on Osteoblastogenesis and Bone Mineral Density in Mice
Kyoung Min Kim, Hyun-Jin Jin, Seo Yeon Lee, Hyo Jin Maeng, Gha Young Lee, Tae Jung Oh, Sung Hee Choi, Hak Chul Jang, Soo Lim
Endocrinology and Metabolism.2017; 32(3): 389. CrossRef - Effects of Lobeglitazone, a Novel Thiazolidinedione, on Bone Mineral Density in Patients with Type 2 Diabetes Mellitus over 52 Weeks
Soo Lim, Kyoung Min Kim, Sin Gon Kim, Doo Man Kim, Jeong-Taek Woo, Choon Hee Chung, Kyung Soo Ko, Jeong Hyun Park, Yongsoo Park, Sang Jin Kim, Hak Chul Jang, Dong Seop Choi
Diabetes & Metabolism Journal.2017; 41(5): 377. CrossRef
- The modulation effects of plant‐derived bioactive ingredients on chronic kidney disease: Focus on the gut–kidney axis

- Mechanisms of Vascular Calcification: The Pivotal Role of Pyruvate Dehydrogenase Kinase 4
- Jaechan Leem, In-Kyu Lee
- Endocrinol Metab. 2016;31(1):52-61. Published online March 16, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.1.52
- 4,461 View
- 69 Download
- 30 Web of Science
- 28 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Vascular calcification, abnormal mineralization of the vessel wall, is frequently associated with aging, atherosclerosis, diabetes mellitus, and chronic kidney disease. Vascular calcification is a key risk factor for many adverse clinical outcomes, including ischemic cardiac events and subsequent cardiovascular mortality. Vascular calcification was long considered to be a passive degenerative process, but it is now recognized as an active and highly regulated process similar to bone formation. However, despite numerous studies on the pathogenesis of vascular calcification, the mechanisms driving this process remain poorly understood. Pyruvate dehydrogenase kinases (PDKs) play an important role in the regulation of cellular metabolism and mitochondrial function. Recent studies show that PDK4 is an attractive therapeutic target for the treatment of various metabolic diseases. In this review, we summarize our current knowledge regarding the mechanisms of vascular calcification and describe the role of PDK4 in the osteogenic differentiation of vascular smooth muscle cells and development of vascular calcification. Further studies aimed at understanding the molecular mechanisms of vascular calcification will be critical for the development of novel therapeutic strategies.
-
Citations
Citations to this article as recorded by- Gamut of glycolytic enzymes in vascular smooth muscle cell proliferation: Implications for vascular proliferative diseases
Ankan Sarkar, Sandip V. Pawar, Kanwaljit Chopra, Manish Jain
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2024; 1870(3): 167021. CrossRef - MAPK14 as a key gene for regulating inflammatory response and macrophage M1 polarization induced by ferroptotic keratinocyte in psoriasis
Lin Zhou, Yingdong Zhong, Chaowei Li, Yu Zhou, Xi Liu, Lincai Li, Zhengwei Zou, Zhihui Zhong, Junsong Ye
Inflammation.2024;[Epub] CrossRef - Pyruvate dehydrogenase kinase 4 promotes osteoblastic potential of BMP9 by boosting Wnt/β-catenin signaling in mesenchymal stem cells
Yuan-Yuan Yang, Hong-Hong Luo, Yi-Xuan Deng, Xin-Tong Yao, Jie Zhang, Yu-Xi Su, Bai-Cheng He
The International Journal of Biochemistry & Cell Biology.2023; 154: 106341. CrossRef - lncRNA MEG3 Promotes PDK4/GSK-3β/β-Catenin Axis in MEFs by Targeting miR-532-5p
Yuan-Yuan Yang, Yi-Xuan Deng, Xin-Tong Yao, Hong-Hong Luo, Wen-Ge He, Xuan-Ling Cao, Rong-Chun Chen, Bai-Cheng He, Hai-Tao Jiang, Jing Wang, Sedat Kacar
Oxidative Medicine and Cellular Longevity.2023; 2023: 1. CrossRef - Mitochondrial dynamics in vascular remodeling and target-organ damage
Tong Zhu, Qingxun Hu, Yanggang Yuan, Huijuan Yao, Jian Zhang, Jia Qi
Frontiers in Cardiovascular Medicine.2023;[Epub] CrossRef - PDK4-dependent hypercatabolism and lactate production of senescent cells promotes cancer malignancy
Xuefeng Dou, Qiang Fu, Qilai Long, Shuning Liu, Yejun Zou, Da Fu, Qixia Xu, Zhirui Jiang, Xiaohui Ren, Guilong Zhang, Xiaoling Wei, Qingfeng Li, Judith Campisi, Yuzheng Zhao, Yu Sun
Nature Metabolism.2023; 5(11): 1887. CrossRef - Identification of PDK4 as Hub Gene for Diabetic Nephropathy Using Co-Expression Network Analysis
Yuanyuan Han, Liangzi Jin, Liangzhi Wang, Lan Wei, Chao Tu
Kidney and Blood Pressure Research.2023; 48(1): 522. CrossRef - Flavocoxid Ameliorates Aortic Calcification Induced by Hypervitaminosis D3 and Nicotine in Rats Via Targeting TNF-α, IL-1β, iNOS, and Osteogenic Runx2
Ahmed E. Amer, George S. G. Shehatou, Hassan A. El-Kashef, Manar A. Nader, Ahmed R. El-Sheakh
Cardiovascular Drugs and Therapy.2022; 36(6): 1047. CrossRef - Diabetic mellitus, vascular calcification and hypoxia: A complex and neglected tripartite relationship
Xue-Jiao Sun, Nai-Feng Liu
Cellular Signalling.2022; 91: 110219. CrossRef - Insights Into the Role of Mitochondria in Vascular Calcification
ZL Zeng, Qing Yuan, Xuyu Zu, Jianghua Liu
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Induced pluripotent stem cell-derived smooth muscle cells to study cardiovascular calcification
Samantha K. Atkins, Abhijeet R. Sonawane, Romi Brouwhuis, Johana Barrientos, Anna Ha, Maximillian Rogers, Takeshi Tanaka, Takehito Okui, Shiori Kuraoka, Sasha A. Singh, Masanori Aikawa, Elena Aikawa
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Phenotypic plasticity of vascular smooth muscle cells in vascular calcification: Role of mitochondria
Yan Zhong Liu, Zong Xiang Li, Lin Lin Zhang, Dan Wang, Yi Ping Liu
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Febuxostat attenuates vascular calcification induced by vitamin D3 plus nicotine in rats
Ahmed E. Amer, Ahmed R. El-Sheakh, Mohamed F. Hamed, Hassan A. El-Kashef, Manar A. Nader, George S.G. Shehatou
European Journal of Pharmaceutical Sciences.2021; 156: 105580. CrossRef - Mitochondria and traffic-related air pollution linked coronary artery calcification: exploring the missing link
Bhavana Sivakumar, Gino A. Kurian
Reviews on Environmental Health.2021; 36(4): 545. CrossRef - Mitochondria Homeostasis and Vascular Medial Calcification
Min li, Yi Zhu, Sandip Kumar Jaiswal, Nai-Feng Liu
Calcified Tissue International.2021; 109(2): 113. CrossRef - POSTN promotes diabetic vascular calcification by interfering with autophagic flux
Xue-Jiao Sun, Wen-Qi Ma, Yi Zhu, Nai-Feng Liu
Cellular Signalling.2021; 83: 109983. CrossRef - The MAMs Structure and Its Role in Cell Death
Nan Wang, Chong Wang, Hongyang Zhao, Yichun He, Beiwu Lan, Liankun Sun, Yufei Gao
Cells.2021; 10(3): 657. CrossRef - Pyruvate dehydrogenase kinases (PDKs): an overview toward clinical applications
Xiuxiu Wang, Xiaoyue Shen, Yuting Yan, Hongmin Li
Bioscience Reports.2021;[Epub] CrossRef - Label-Free Multiphoton Microscopy for the Detection and Monitoring of Calcific Aortic Valve Disease
Ishita Tandon, Kyle P. Quinn, Kartik Balachandran
Frontiers in Cardiovascular Medicine.2021;[Epub] CrossRef - Vascular Calcification—New Insights into Its Mechanism
Sun Joo Lee, In-Kyu Lee, Jae-Han Jeon
International Journal of Molecular Sciences.2020; 21(8): 2685. CrossRef - Osteocalcin Regulates Arterial Calcification Via Altered Wnt Signaling and Glucose Metabolism
Nabil A Rashdan, Alisia M Sim, Lin Cui, Kanchan Phadwal, Fiona L Roberts, Roderick Carter, Derya D Ozdemir, Peter Hohenstein, John Hung, Jakub Kaczynski, David E Newby, Andrew H Baker, Gerard Karsenty, Nicholas M Morton, Vicky E MacRae
Journal of Bone and Mineral Research.2020; 35(2): 357. CrossRef - The role of mitochondria in vascular calcification
Pengbo Wang, Naijin Zhang, Boquan Wu, Shaojun Wu, Ying Zhang, Yingxian Sun
Journal of Translational Internal Medicine.2020; 8(2): 80. CrossRef - Cerebral blood flow in dystonia due to pantothenate kinase-associated neurodegeneration
Peter Stoeter, Pedro Roa-Sanchez, Cesar F Gonzalez, Herwin Speckter, Jairo Oviedo, Pamela Bido
The Neuroradiology Journal.2020; 33(6): 479. CrossRef - PDK4 promotes vascular calcification by interfering with autophagic activity and metabolic reprogramming
Wen-Qi Ma, Xue-Jiao Sun, Yi Zhu, Nai-Feng Liu
Cell Death & Disease.2020;[Epub] CrossRef - Restoring mitochondrial biogenesis with metformin attenuates β-GP-induced phenotypic transformation of VSMCs into an osteogenic phenotype via inhibition of PDK4/oxidative stress-mediated apoptosis
Wen-Qi Ma, Xue-Jiao Sun, Ying Wang, Yi Zhu, Xi-Qiong Han, Nai-Feng Liu
Molecular and Cellular Endocrinology.2019; 479: 39. CrossRef - Salusin-β Promotes Vascular Calcification via Nicotinamide Adenine Dinucleotide Phosphate/Reactive Oxygen Species-Mediated Klotho Downregulation
Haijian Sun, Feng Zhang, Yu Xu, Shuo Sun, Huiping Wang, Qiong Du, Chenxin Gu, Stephen M. Black, Ying Han, Haiyang Tang
Antioxidants & Redox Signaling.2019; 31(18): 1352. CrossRef - Fibroblast Growth Factor 21 (FGF21) Promotes Formation of Aerobic Myofibers via the FGF21‐SIRT1‐AMPK‐PGC1α Pathway
Xinyi Liu, Yongliang Wang, Liming Hou, Yuanzhu Xiong, Shuhong Zhao
Journal of Cellular Physiology.2017; 232(7): 1893. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef
- Gamut of glycolytic enzymes in vascular smooth muscle cell proliferation: Implications for vascular proliferative diseases


 KES
KES

 First
First Prev
Prev



